Sulfate respiration (sulfate reduction) and blue tide
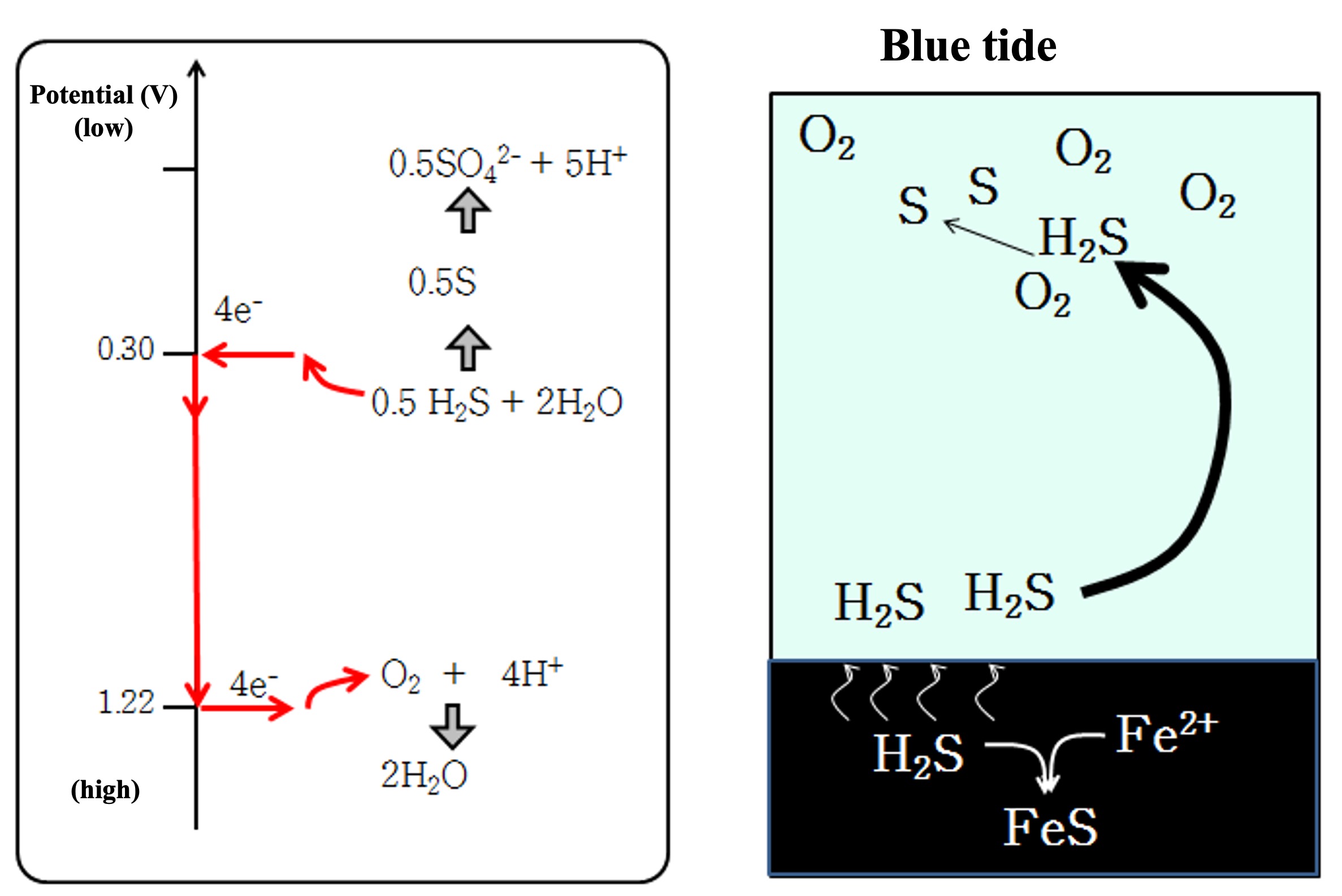
Now, when oxygen is depleted in the water, microorganisms (nitrate-reducing bacteria) appear that use nitric acid as an oxidant. When nitric acid also runs out, the absolutely anaerobic bacteria sulfate-reducing bacteria appear. As explained earlier, sulfate reduction occurs in an environment rich in organic matter and sulfate ions, where oxygen supply from the outside world is severely limited. Because seawater is rich in sulfate ions, sulfate reduction occurs in marine sediments at depths ranging from a few centimeters to several tens of centimeters below the surface. When marine sediments are sampled, such depths may reveal a hydrogen sulfide odor and a layer of black iron sulfide (FeS) formed by the reaction of hydrogen sulfide and iron ions.
Where there is a large amount of organic sediment and stagnant bottom water, the bottom water can become anoxic. Sulfate reduction occurs near the surface of marine sediments, producing hydrogen sulfide. When this highly poisonous hydrogen sulfide accumulates in anoxic bottom waters, the marine environment deteriorates significantly. Furthermore, when bottom waters rise due to weather disturbances and meet oxygen in the surface layer, hydrogen sulfide is reduced to form single sulfur particles. These sulfur particle colloids scatter light, making them appear blue when viewed from the sea. This is the blue tide. The surface water in which a blue tide occurs is also hypoxic, which can cause fish kills and other damage. The single sulfur particles are eventually oxidized back to sulfate ions.
Up to this point, we have been calculating the total difference in the standard Gibbs energy of formation (⊿∑Gf0) before and after a chemical reaction, using the respiration reaction of living organisms as an example. I hope you are now a little more familiar with it.
In the next course, we will use ⊿∑Gf0 to calculate the concentration of each substance when the chemical reaction is complete (in equilibrium). This is where calculating chemical equilibrium comes into play in analytical chemistry.
In the Department of Marine Biology of the Faculty of Fisheries Sciences, when dealing with analytical chemistry, it is sufficient to remember and use the basic equations of chemical equilibrium in the following courses.