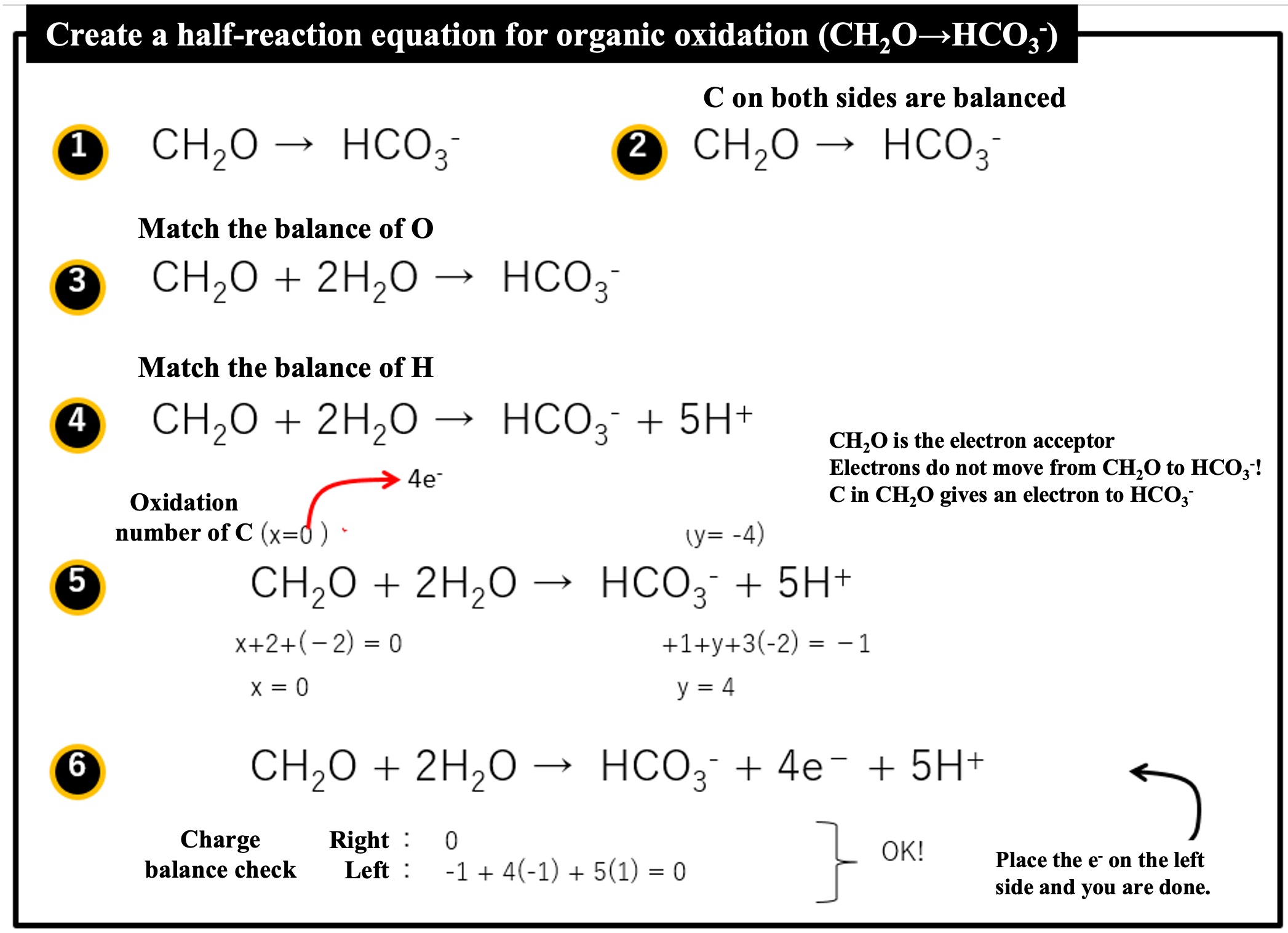
How to make a half-reaction equation (example of organic oxidation (CH2O → HCO3-)
We make a half-reaction equation in which formaldehyde (CH
2O) in water is oxidized to produce bicarbonate ions (HCO
3-). In the previous example, we made an equation that produces carbon dioxide (CO
2), but carbon dioxide is immediately converted to hydrogen carbonate ions in water, so this half-reaction may be occurring in vivo.
① We want to find the half-reaction equation where CH2O is oxidized to HCO3-. Connect it with →.
② Match the C of both sides (originally, the C amounts are the same).
③ Match the balance of O and H. First, to match O, we put 2H2O on the left side.
④ Next, to match H, place 5H+ on the right side.
⑤ To examine the electron transfer, we note the oxidation number of C. Let the oxidation number of C in CH2O be x (0 = x +2 -2) and the oxidation number of C in HCO3- be y (-1 = 1+y-6). The electron transfer is 4e- since the oxidation number of C in CH2O changes from 0 to +4 in HCO3-.
⑥ Add the amount of electrons (4e-) to be transferred. Check the charge balance between the left and right sides. Both sides are 0, so it is OK. (If 4e- is placed on the left side, the charge on the left side will be -4 and that on the right side will be +4.) To make e- on the left side, both sides are swapped and connected by an equal sign. (In the above case, e- is placed on the right side, so swap the left and right sides.)