Again, we note the half-reaction equation and Nernst equation for the dissolution of iron hydroxide.
Dissolution of iron hydroxide ( half-reaction equation): Fe(OH)3 + 3 H+
+ e- = Fe2+ + 3
H2O
Nernst equation
E = E0 -RT/(F)・Ln { [Fe2+] /( [H+]3
・[Fe(OH)3])
=
0.969 -
0.024387・Ln{ [Fe2+] / [Fe(OH)3]} - 0.168459pH
(As a physical chemistry promise, in a solution reaction of a solid (Fe(OH)3), the solid concentration = 1, given that there is a sufficient amount of that solid.)
The condition for separating the Fe2+ and Fe(OH)3 abundance ratios [Fe2+] / [Fe(OH)3] = 1,
([Fe(OH)3] = 1 (mol/L), so when [Fe2+] = 1 (mol/L))
E = 0.969 - 0.168459pH.
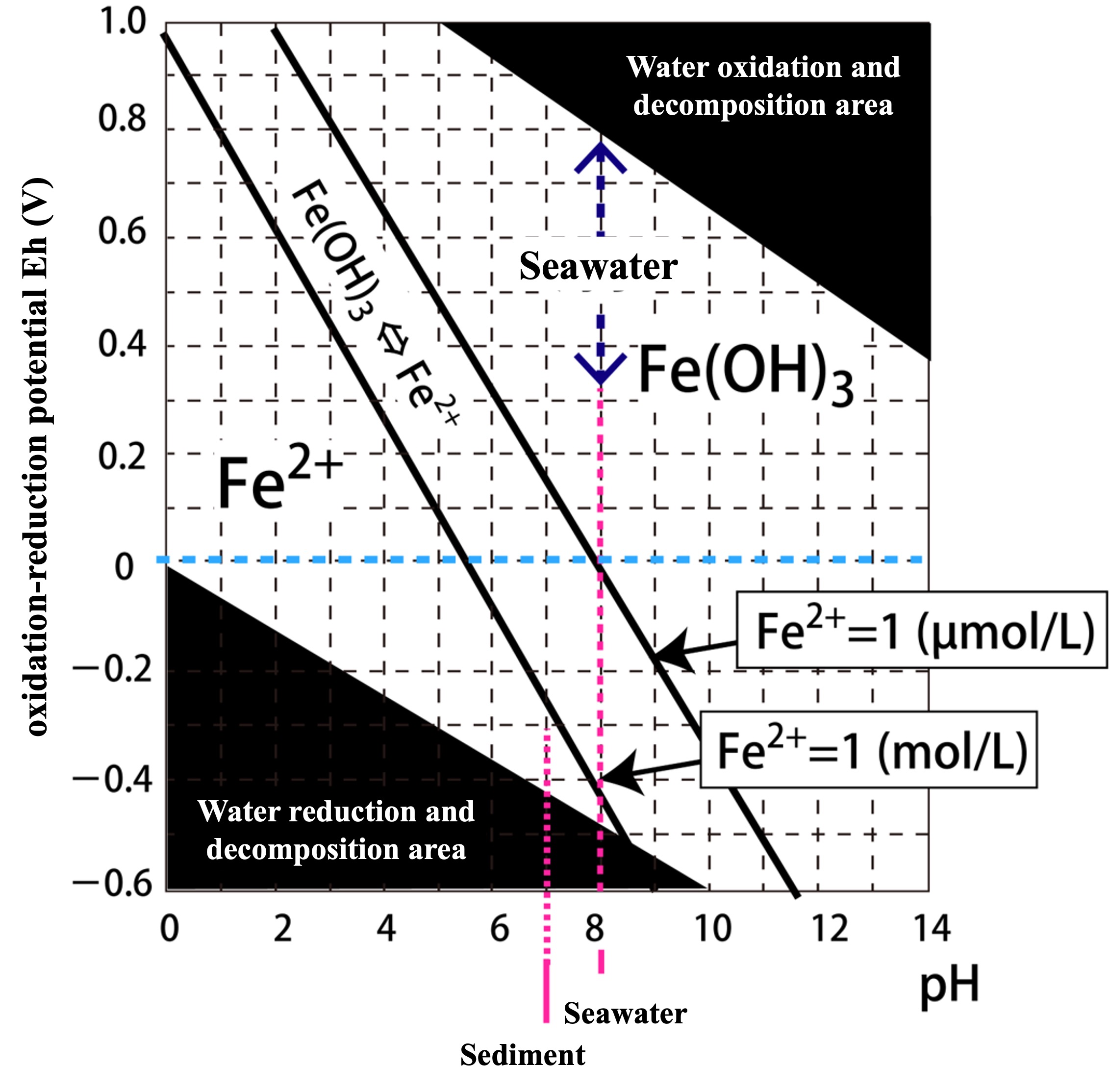
Taking pH on the horizontal axis and E on the vertical axis, this relational equation is depicted in the figure below.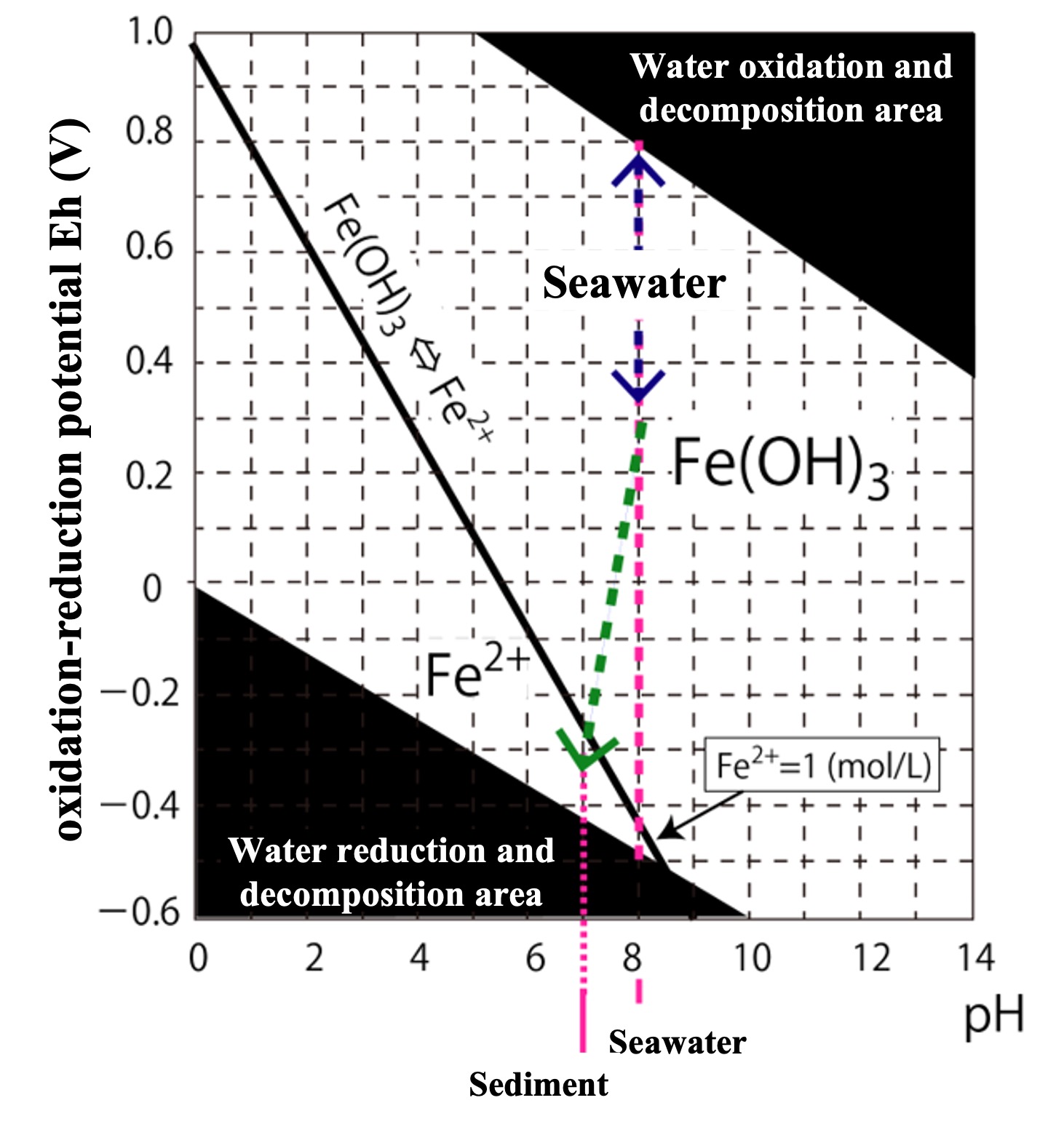
The area above the black bold line in the boundary condition (Fe(OH)3 ⇔ Fe2+) means that solid Fe(OH)3 is present, and Fe2+ has reached dissolution equilibrium at less than 1 mol/L.
So what would the line be for [Fe2+] < 1 mol/L?
The line at the dissolution equilibrium concentration [Fe2+] = 1 µmol/L is added to the figure below. You can see a large shift.