We now want to calculate the energy that can be acquired by the respiration reaction of the organism. First, we will use a simple reaction equation as an example to calculate the energy difference before and after the reaction.
The total energy possessed by a substance is called enthalpy. The total energy (enthalpy) is divided into bound energy (entropy) and Gibbs free energy.
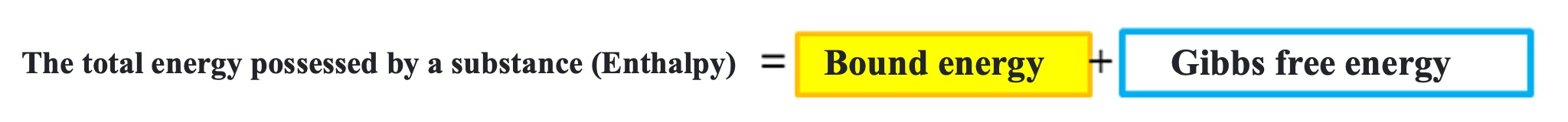
The binding energy at standard conditions (1 atm, 25°C) is called the standard entropy or standard gibbs energy of formation.
You are now asked to calculate the "Total difference in standard Gibbs energy of formation before and after chemical reaction". The long words are again 100% up to your mind. Let's get used to it by solving examples.