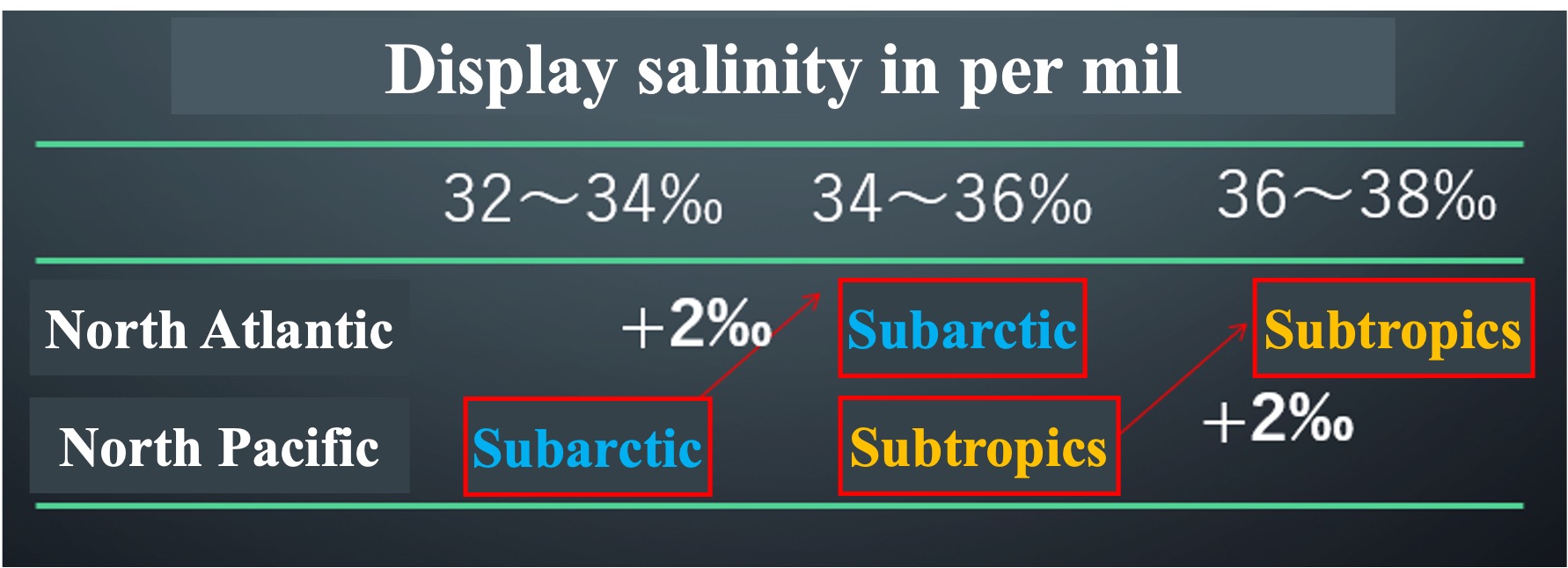
Compare the salinity of the surface layer of the North Pacific Ocean and the North Atlantic Ocean
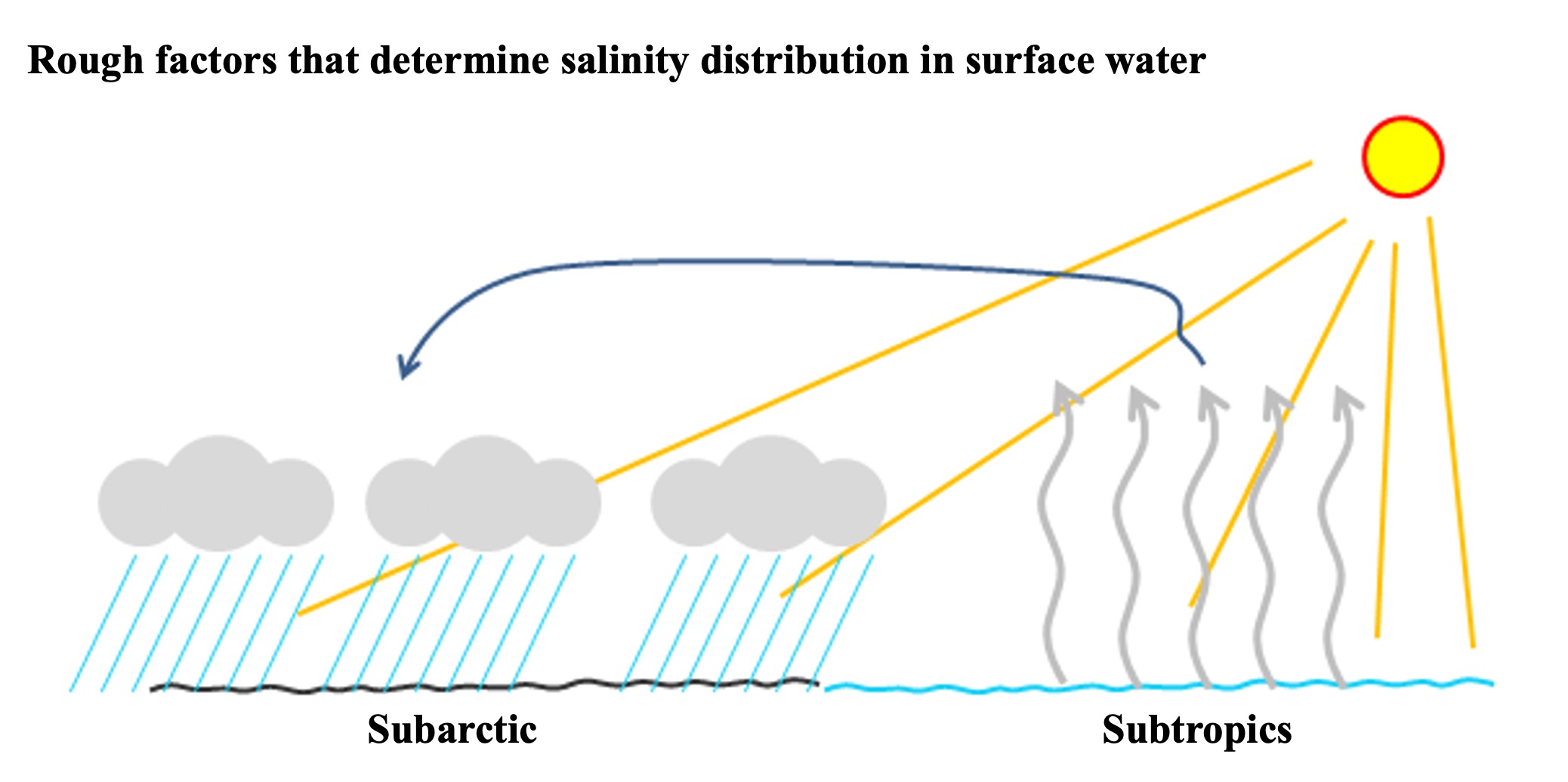
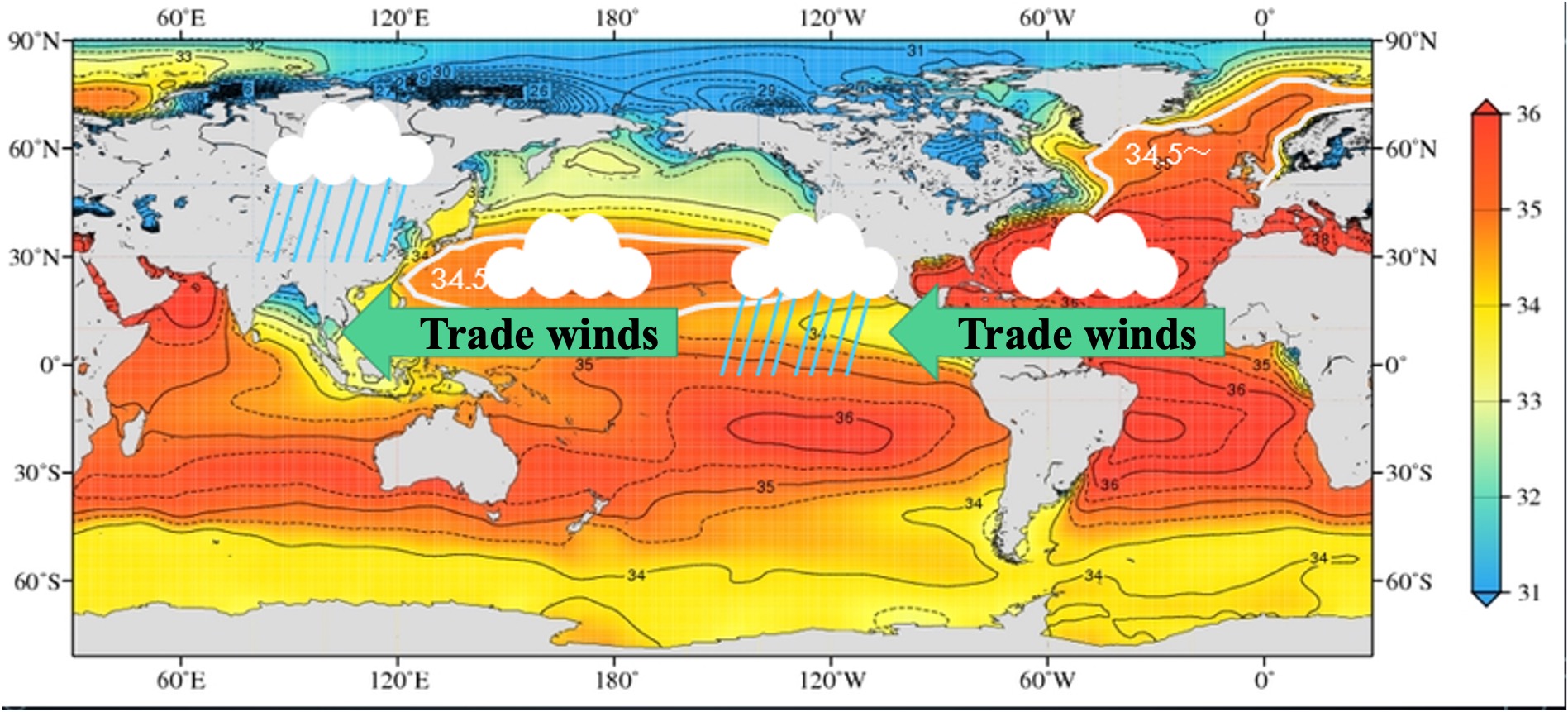
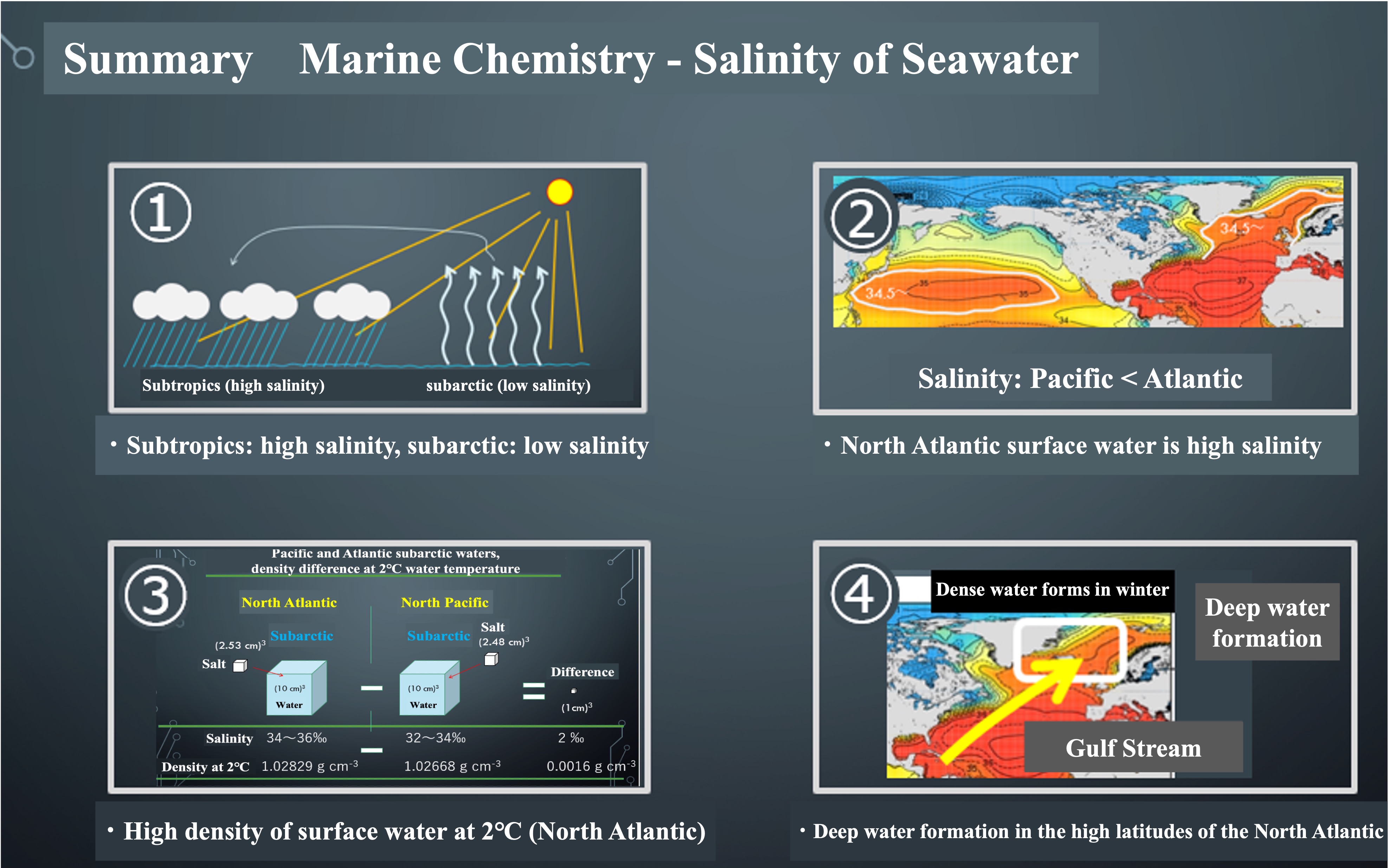
Salinity in seawater is expressed in per thousandths of a percent. We usually use percentages in hundredths, but salinity in oceanography is expressed in thousandths of a permil. The Atlantic Ocean is two to three per mils saltier than the Pacific Ocean. The Atlantic Ocean is more salty than the North Atlantic and the North Pacific when compared between the subarctic and arctic zones. The Atlantic Ocean is also more salty than the subtropical Atlantic Ocean. Let's visualize the difference in salt content.
1000 g approx. 1 L of seawater with 34 g of salt is 34‰. The salinity of the Atlantic subarctic surface layer is 34-36‰, so 1 L of water measuring 10 cm x 10 cm x 10 cm contains about 35 g of salt. The salinity of the Pacific subarctic surface layer is 32-34‰, so 1 L of water contains about 33 g of salt. What is the difference in the amount of salt between the Atlantic and Pacific Oceans?
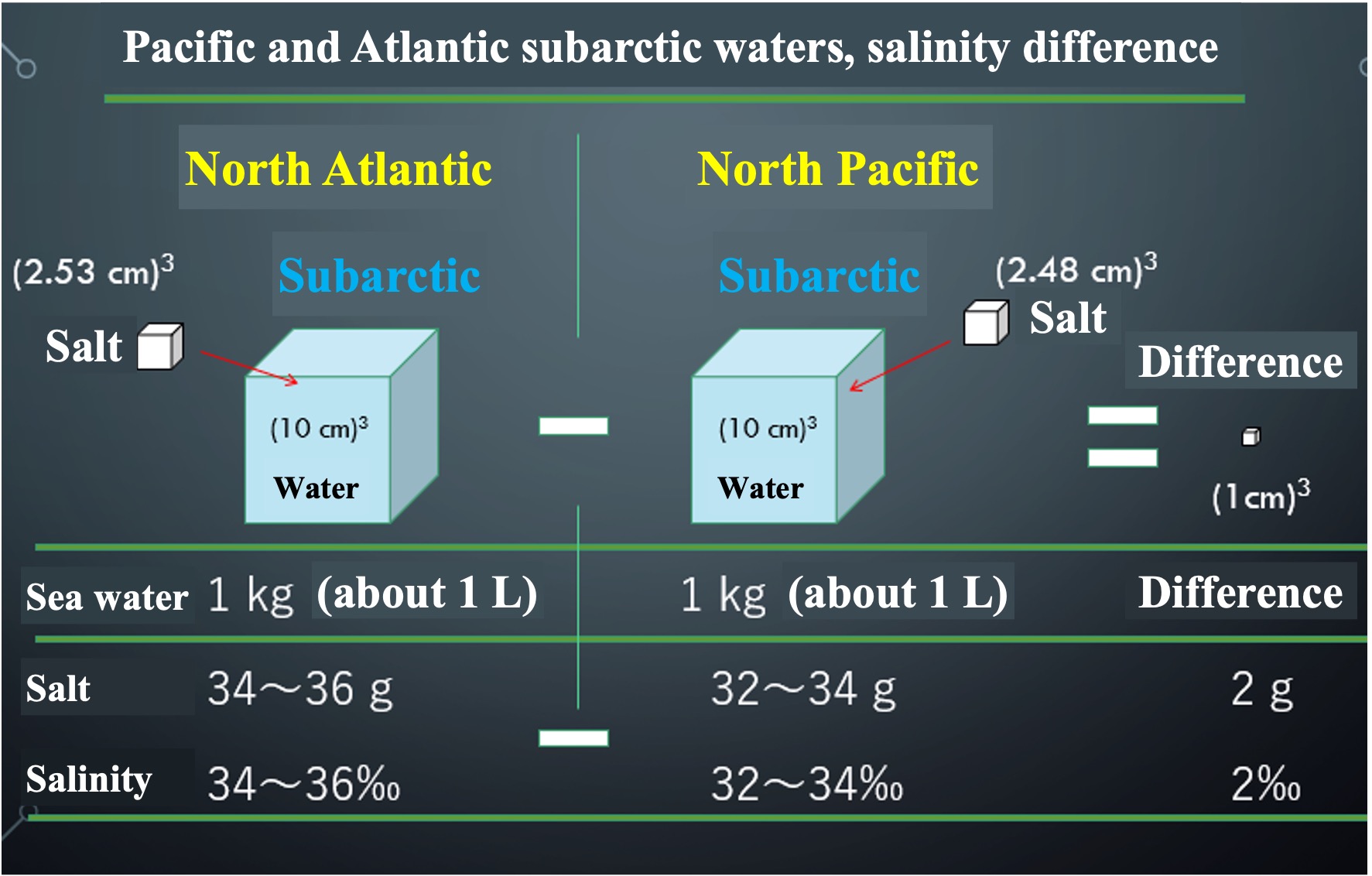
If we take the difference in the amount of salt between the Atlantic and Pacific Oceans, the difference in salinity is 2‰ and the difference in weight is 2 g. The 2 g weight difference is equivalent to 1 cubic centimeter of salt in volume. This difference in the amount of salt contained is what creates the ocean general circulation, which is driven by the density difference of seawater.
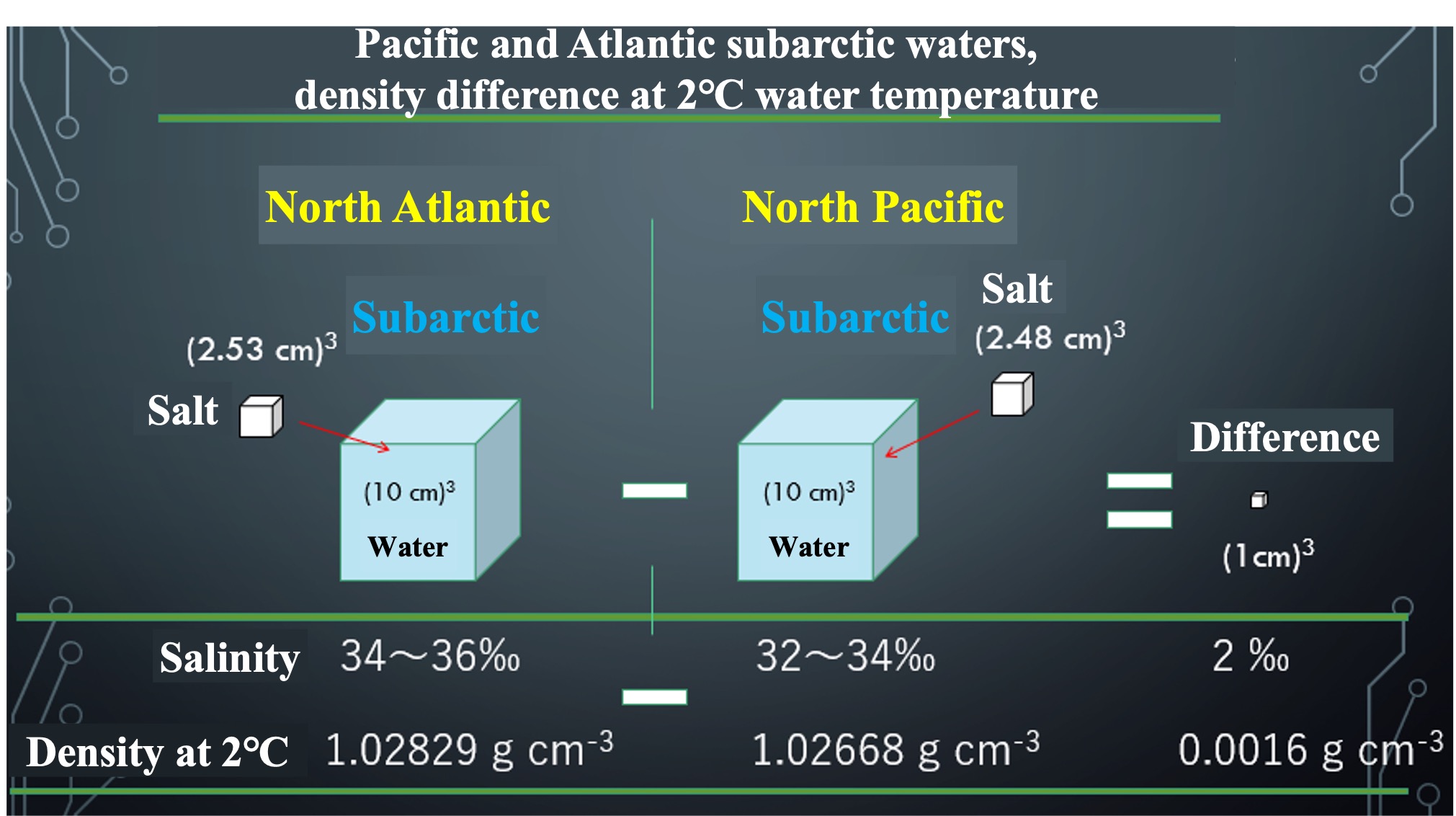
Calculate the density. In the subarctic zone of the North Atlantic and North Pacific, sea water is cooled in winter. Suppose the water temperature drops to 2°C. Calculated density at that time is 1.02829 g cm-3 for the Atlantic Ocean. The Pacific is 1.02668 g cm-3. The difference is only 0.0016 g cm-3.
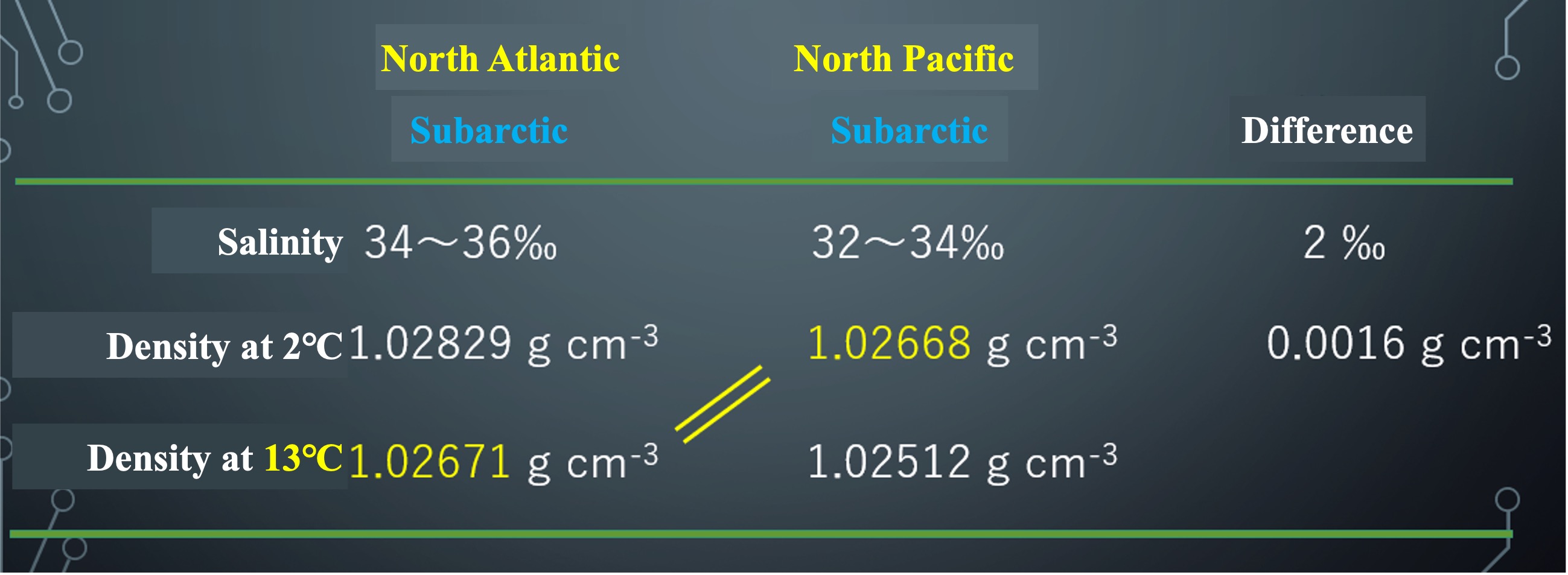
Surface water in the subarctic zone can rise to a water temperature of about 13°C in summer. The density at that time was determined. The density of water at 13°C in the Atlantic subarctic is 1.02671 g cm-3, which is the same as the density of water at 2°C in the Pacific subarctic. Water in the Pacific cannot be heavier than water in the Atlantic, no matter how much it is cooled. When surface water cools rapidly in the subarctic North Atlantic and becomes denser and sinks to the depths, the deeper water is pushed down to the deeper layers of the Pacific Ocean in a roundabout way.
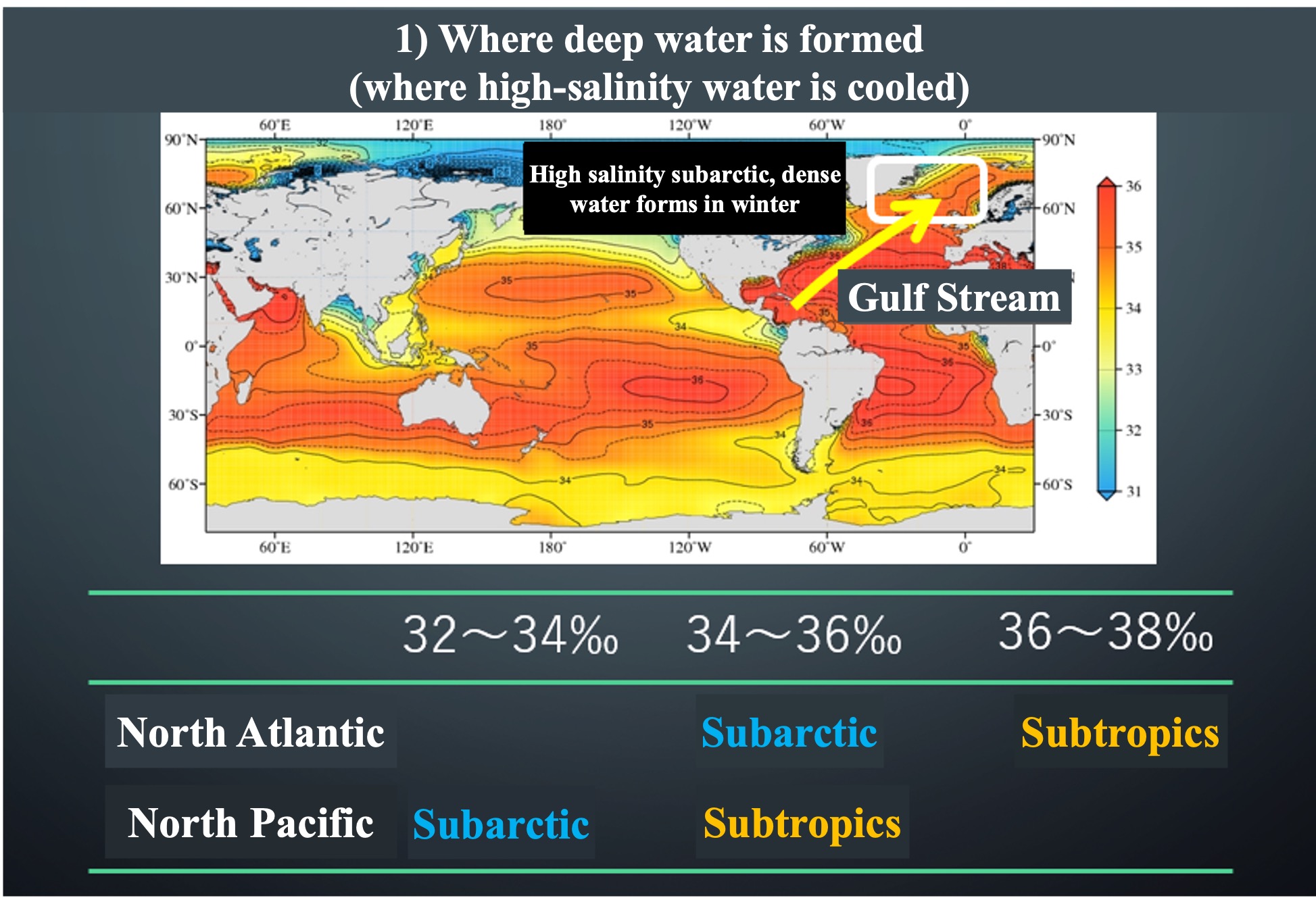
Once again, let's look at the salinity distribution at the ocean surface. The high-salinity subtropical Atlantic waters are carried to higher latitudes by the strong currents of the Gulf Stream. When this high-salinity surface water cools rapidly near the North Pole, it creates high-density water that gravity falls to the deeper ocean layers. As the water is supplied to the deeper layers each winter, it is pushed away. This is the start of the deep circulation. The deep circulation will be explained in the next article.