The
first step o developing an immunochemical detection and measurement method is
to prepare a highly pure protein of interest as an "immune antigen."
The process of increasing the purity of a target protein from a sample
containing multiple proteins is called "purification."
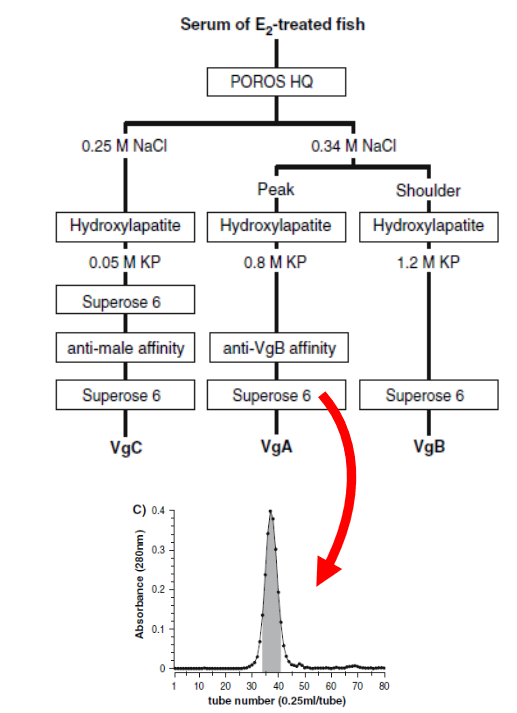
Example
of purification of oogenesis
related protein:
The
yolk protein precursor is called vitellogenin (vitellogenin: Vg).
The figure on the left is a flowchart of the purification of three types of Vg
from the blood of a mullet (Mugil
cephalus)
to which female hormones have been administered. For
example, in the case of VgA, we first use one POROS HQ of the ion-exchange
column, followed by a negative affinity column combining the hydroxyapatite
column and VgB acid, and finally one Superose 6 of the gel filtration column,
which is purified through
a total of four-column
chromatographies.
Example
of the chromatogram of gel filtration column:
Gel
filtration column is a method to separate based on the differences in the
molecules of protein. The figure on the left is a VgA
elution chromatogram with the vertical axis indicating the absorbance value and
the horizontal axis indicating the elution fraction number. Generally
speaking, if you get one symmetrical peak, we
think it is highly purified.